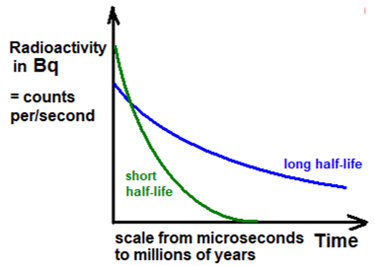
Half-life, in radioactivity, is the interval of time required for one-half of the atomic nuclei of a radioactive sample to decay (change spontaneously into other nuclear species by emitting particles and energy), or, equivalently, the time interval required for the number of disintegrations per second of a radioactive material to decrease by one-half.
Often isotopes are referred to as short-lived or long-lived meaning sort of long half-lives. While there is no definition of the distinction short will usually be less than 100 years and long longer than this.
Further details on radioactive decay are give on the page:
Essential of Nuclear Physics