Isotopes
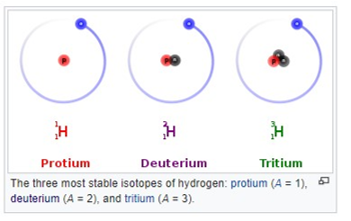
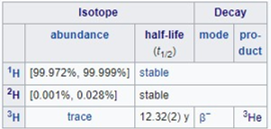
All isotopes of a given element have almost the same chemical properties. Some isotopes are stable and others are unstable and undergo radioactive decay. These are sometimes called radioisotopes.
Hydrogen is the only element whose isotopes have different names that remain in common use. Deuterium has a nucleus with one proton and one neutron and tritium has a nucleus with one proton and two neutrons. The symbols D and T are sometimes used for deuterium and tritium.
Further details on isotopes are give on the page: Essential of Nuclear Physics